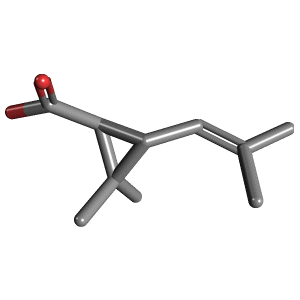
(+)-trans-Chrysanthemic acid
(+)-trans-Chrysanthemic acid (2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylic acid, 2,2-Dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylic acid, CAS#4638-92-0), is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids. One of the four stereoisomers, (1R,3R)- or (+)-trans-chrysanthemic acid (pictured), is the acid-derived group of pyrethrin I, which occurs naturally in the seed cases of Chrysanthemum cinerariaefolium. Many synthetic pyrethroids, for example the allethrins, are esters of all four stereoisomers.
Biosynthesis: Chrysanthemic acid is derived from its pyrophosphate ester, which in turn is produced naturally from dimethylallyl diphosphate:
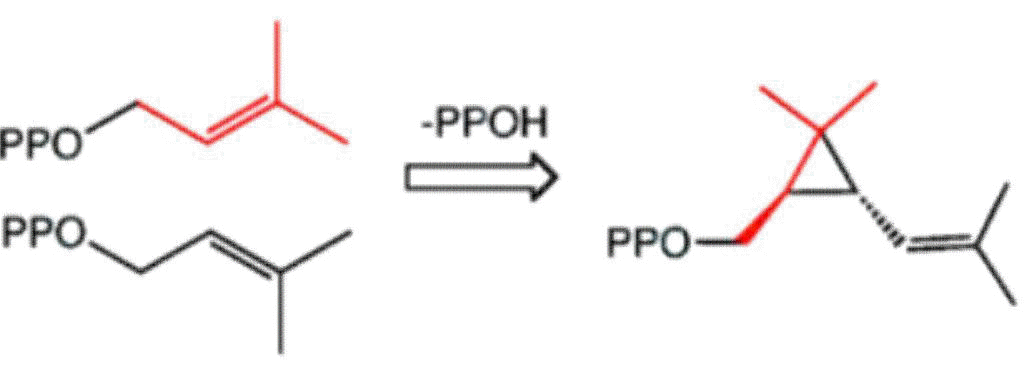
Synthesis: Chrysanthemic acid is produced industrially in a cyclopropanation reaction of a diene as a mixture of cis- and trans isomers, followed by hydrolysis of the ester:
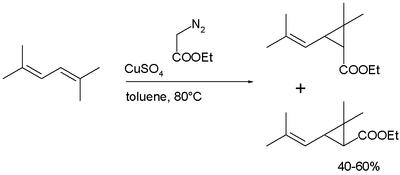
Many pyrethroids are accessible by re-esterification of chrysanthemic acid ethylester.
Uses
Package & Transporttt
Package Size: 50kg/drum
UN Number: 3080
Hazard Class: 6.1
Notice
1. Store in a cool, dry place.
2. Keep container closed when not in use. Corrosives area.
3. Keep containers tightly closed.
(+)-trans-Chrysanthemic acid
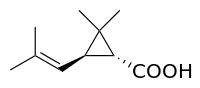
|
|
| Molecular formula | C10H16O2 |
| Molar massss | 168.23 g/mol |
IUPAC Name
Identifiers
SMILES[hide]
InChI[hide]
Key: XLOPRKKSAJMMEW-JGVFFNPUSA-N
Properties
| Appearance | Clear viscous liquid |
| Density | 1.01 g/cm3 |
| Melting point | 17-21℃ |
| Boiling point | 80 ℃ (0.5 torr) |
| Vapour pressure | 0.00925 mmHg |
| Solubility in water | 164 mg/L |
Hazards[hide]
| MSDS | ChrysanthemicAcid_MSDS.pdf |
| R-phrases | R36/37/38 |
| S-phrases | S26, S37/39 |
|
Main hazards | XI |
| Flash point | 119℃ |
Supplementary data[hide]
| Spectral data | UV, IR, NMR, MS |
| TDS | ChrysanthemicAcid_TDS.pdf |
| Others Data | Analysis_report; Toxic_report; Etoxic_report; SGS_report, etc. |

 English
English 中文简体
中文简体


 Arabic
Arabic  French
French  Italian
Italian  German
German  Japanese
Japanese  Korean
Korean  Portuguese
Portuguese  Russian
Russian  Spanish
Spanish 