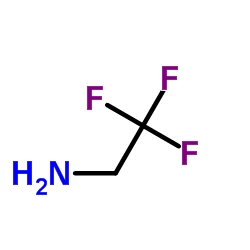
2,2,2-Trifluoroethylamine
Trifluoroethylamine ,
2,2,2-Trifluoroethanamine, '2,2,2-trifluoro-ethanamin', 'Ethylamine, 2,2,2-trifluoro-',
is the a volatile organic compound, was tested using corona discharge ion mobility spectrometry with orthogonal acceleration time of flight mass spectrometry (CD IMS-oaTOF), with the formula CF3CH2NH2. This
colorless liquid with unpleasant gases, storage need low temperature, protected from light.
As pesticide intermediate synthetic insecticides, miticides, ovicide, synthetic human, plant or seed fungicides, herbicides and synthetic key intermediate;
As pharmaceutical intermediates, it can be prepared by 1,2-substituted guanidine and derivatives thereof.
Trifluoroethylamine be used to prepare double-substituted aniline derivatives. As the catalyst, it is for the weakly basic amine catalyst, through the formation of ammonium ions to catalyze the removal of the reaction α protons.
Uses
It can be synthesized active good insecticide, miticide, ovicide, synthetic human, plant or seed fungicides, and synthetic herbicides.
2 As pharmaceutical intermediates
1,2-substituted biguanide may be prepared and derivatives thereof, this material is used as a medicine for treating diabetes. Guanidine derivatives obtained by the trifluoroethylamine is a good inhibition of gastric acid secretion of pharmaceutical intermediates. Trifluoroethylamine be used to prepare double-substituted aniline derivatives, for the treatment of central nervous system, cardiovascular system, respiratory system, urinary system, digestive system, endocrine system diseases. Trifluoro-ethylamine was used to prepare a medical fungicides.
3 As catalyst
As a weakly basic nucleophiles biphenyl-dienone catalytic hydration reaction of biphenyl acetic acid. Do β, γ-unsaturated enone isomerization of an α, β-unsaturated enone of a catalyst, such as 3-methyl-3-cyclohexenone with trifluoroethylamine effect by a double bond to form an intermediate Schiff Migration a base, and then isomerized methyl-2-cyclohexenone. As acetylacetone alcohol, aldehyde removal catalyst. As the catalytic core ketal pro-catalyst β- (e.g. hydroxymethyl naphthalene alkanone) dehydration reaction. Formed during the reaction imino ions. The alkyne (l- octyne, 4-nitrophenyl acetylene, etc.) as catalyst hydrogen shift reaction. Made weakly basic catalyst at one and sodium cyanoborohydride prepared by reacting isopropanol. As weakly basic amine catalyst, to catalyze the formation of ammonia ions removal reaction α protons.
4 As nucleophile
As a nucleophile with bis (trifluoromethyl) difluoride sulfoxide and the reaction with quinoline derivative (e.g., benzene substituted quinoline carboxylate 6,8) nucleophilic aminolysis performed.
5 Other Applications
With diaryl disulfide electrically oxidation condensation of N- (2,2,2- trifluoro-ethylene) - sulfenamide, the latter for the synthesis of trifluoromethyl amines, amino ketone and amino alkanoic acid ester. Trifluoroethylamine and branched chloromethyl polystyrene resin reaction product can be used in Mem-field synthesis. Cyclotriphosphazene cyanide derivatives trifluoroethylamine and HCH cyanide reaction triphosphate synthesis. For the preparation of 2,2,2-trifluoroethanol. Preparation for inhibiting aromatic hydroxamic acid-containing metalloprotease activity sulfone group. And PMMA (polymethyl methacrylate) is used to heat a polymer prepared by reacting an optical waveguide communications and electronic aspects. And silicon chloride synthesis silazane (CF3CH2NH) 4Si, silazane feature is represented by chromatography. Silazane and Me3Al reaction product having a triclinic crystal structure.
Package & Transport
Package Size: 200kg/drum
UN Number: UN 2733 3/PG 2
WGK Germany : 3
HazardClass : 3
PackingGroup : II
Notice
1. Keep away from heat, sparks, and flame. Flammables-area.
2. Keep container closed when not in use. Corrosives area.
3. Keep containers tightly closed.
2,2,2-Trifluoroethylamine
  |
|
| Molecular formula | C2H4F3N (CF3CH2NH2) |
| Molar mass | 99.06 g/mol |
IUPAC Name
Identifiers
| CAS number | 753-90-2 |
| EC number | 212-041-3 |
| ChemSpider | 9390 |
| RTECS number | KS0175000 |
| PubChem | 9773 |
| Jmol-3D images | Image |
SMILES[show]
InChI[show]
Properties
| Appearance | Colorless liquid |
| Density | 1.24 g/cm3 |
| Boiling point | 37-38℃ |
| Vapour pressure | 7.6 psi |
| Refractive Index | nD20 = 1.3~1.302 |
| Log Kow | 0.24 |

 English
English 中文简体
中文简体


 Arabic
Arabic  French
French  Italian
Italian  German
German  Japanese
Japanese  Korean
Korean  Portuguese
Portuguese  Russian
Russian  Spanish
Spanish 