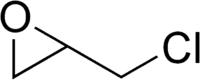
Epichlorohydrin
Epichlorohydrin (ECH, Chloromethyloxirane, 1-chloro-2,3-epoxypropane, γ-chloropropylene oxide, glycidyl chloride, CAS#106-89-8) is an organochlorine compound and an epoxide. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents. Epichlorohydrin is a highly reactive compound and is used in the production of glycerol, plastics, epoxy glues and resins, and elastomers. In contact with water, epichlorohydrin hydrolyzes to 3-MCPD, a carcinogen found in food.
Epichlorohydrin is manufactured:
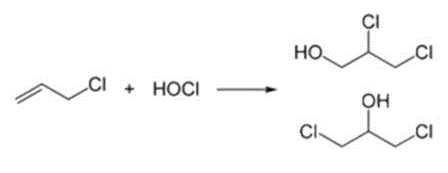
from allyl chloride in two steps, beginning with the hydrochlorination using hypochlorous acid, which affords a mixture of two alcohols:
CH2=CHCH2Cl + HOCl → HOCH2CHClCH2Cl and, or ClCH2CH(OH)CH2Cl
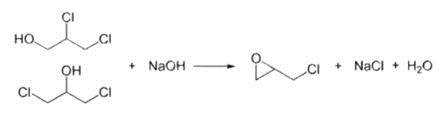
In the second step, this mixture is treated with base to give the epoxide:
HOCH2CHClCH2Cl and, or ClCH2CH(OH)CH2Cl + NaOH → CH2CHOCH2Cl + NaCl + H2O
In this way, more than 800,000 tons of epichlorohydrin are produced annually.
From glycerol
Glycerol is a co-product of biodiesel produced on a large scale. The conversion of glycerol into other building block chemicals is of great interest because glycerol is otherwise hard to dispose of. Dow and Solvay are building glycerol-to-epichlorohydrin (GTE) plants in Shanghai and Thailand, respectively. In Dow's process, glycerol is dichlorinated with hydrogen chloride with the help of a carboxylic acid catalyst. The mixture of dichlorohydroxypropanes is treated with base to form epichlorohydrin.
Uses
Epichlorohydrin is mainly converted to bisphenol A diglycidyl ether, a building block in the manufacture of epoxy resins. It is also a precursor to monomers for other resins and polymers. Another usage is the conversion to synthetic glycerol: CH2CHOCH2Cl + 2 H2O → HOCH2CH(OH)CH2(OH) + HCl However, the rapid increase in biodiesel production, where glycerol is a waste product, has led to a glut of glycerol on the market, rendering this process uneconomic for the mass market. Synthetic glycerol is now used only in sensitive pharmaceutical, technical and personal care applications where quality standards are very high.
Minor and niche applications:
Epichlorohydrin is a versatile precursor in the synthesis of many organic compounds. For example, it is converted to glycidyl nitrate, an energetic binder used in explosive and propellant compositions. The epichlorohydrin is reacted with an alkali nitrate, such as sodium nitrate, producing glycidyl nitrate and alkali chloride. It is used as a solvent for cellulose, resins, and paints, and it has found use as an insect fumigant. Epichlorohydrin is used in paper reinforcement (used for instance in the food industry to manufacture tea bags, coffee filters, and sausage/salami casings) and water purification. An important biochemical application of epichlorohydrin is its use as crosslinking agent for the production of Sephadex size-exclusion chromatographic resins from dextrans.
Package & Transporttt
Package Size: 25kg/drum
UN Number: 2023
Hazard Class: 6.1
Notice
1. Store in a cool, dry place.
2. Keep container closed when not in use. Corrosives area.
3. Keep containers tightly closed.
Epichlorohydrin
  |
|
| Molecular formula | C3H5ClO |
| Molar massss | 92.52 g/mol |
IUPAC Name
Identifiers
| CAS number | 106-89-8 |
| EC number | 203-439-8 |
| ChemSpider | 13837112 |
| RTECS number | TX4900000 |
| UNII | |
| KEGG | C14449 |
| ChEBI | CHEBI:15409 |
| PubChem | 7835 |
| Jmol-3D images | Image 1 |
SMILES[hide]
InChI[hide]
Key: BRLQWZUYTZBJKN-UHFFFAOYSA-N
InChI=1/C3H5ClO/c4-1-3-2-5-3/h3H,1-2H2
Key: BRLQWZUYTZBJKN-UHFFFAOYAY
Properties
| Appearance | Colorless liquid with a slightly irritating |
| Density | 1.181 g/cm3 |
| Melting point | -48 ℃ | Boiling point | 116 ℃ |
| Vapour pressure | 1.6 Kpa |
| Solubility in water | 6 g/100ml |
Hazards[hide]
| MSDS | Epichlorohydrin_MSDS.pdf |
| R-phrases | R10, R23/24/25, R34, R43, R45 |
| S-phrases | S45, S53 |
|
EU classification |
|
| NFPA 704 |
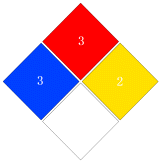 |
| Flash point | 31 ℃ |
Supplementary data[hide]
| Spectral data | UV, IR, NMR, MS |
| TDS | Epichlorohydrin_TDS.pdf |
| Others Data | Analysis_report; Toxic_report; Etoxic_report; SGS_report, etc. |

 English
English 中文简体
中文简体


 Arabic
Arabic  French
French  Italian
Italian  German
German  Japanese
Japanese  Korean
Korean  Portuguese
Portuguese  Russian
Russian  Spanish
Spanish 